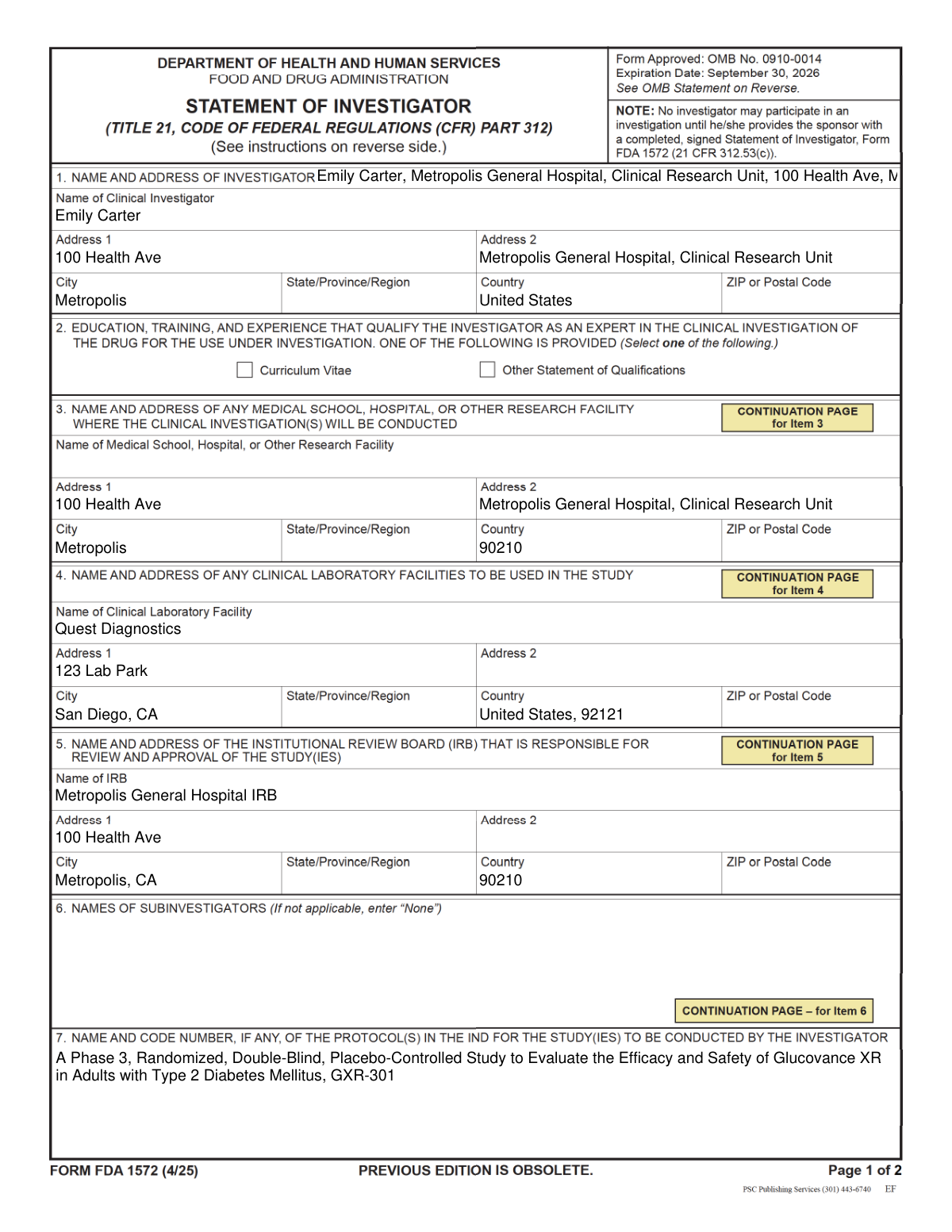
FDA Form 1572, Statement of Investigator Completed Form Examples and Samples
Explore comprehensive, filled-out examples of FDA Form 1572, the Statement of Investigator. Our detailed samples provide a clear guide for clinical researchers on how to correctly complete each section for an Investigational New Drug (IND) application.
FDA Form 1572 Example – Investigational New Drug (IND) Study
How this form was filled:
This is an example of a properly filled FDA Form 1572 for a Phase 3 clinical trial. It details the investigator, Dr. Emily Carter, her clinical site, the protocol for the investigational drug 'Glucovance XR', the responsible Institutional Review Board (IRB), and the list of subinvestigators. The form is signed and dated, signifying the investigator's commitment to adhering to FDA regulations.
Information used to fill out the document:
- Investigator Name and Title: Emily Carter, MD, PhD
- Investigator Address: Metropolis General Hospital, Clinical Research Unit, 100 Health Ave, Metropolis, CA 90210
- Sponsor Name: InnovatePharma Inc.
- Sponsor Address: 500 Discovery Lane, Biotech City, MA 01720
- Investigational Drug Name: Glucovance XR
- Protocol Title: A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Glucovance XR in Adults with Type 2 Diabetes Mellitus
- IND Number: 123,456
- Protocol Number: GXR-301
- Clinical Laboratory: Quest Diagnostics, 123 Lab Park, San Diego, CA 92121
- Institutional Review Board (IRB): Metropolis General Hospital IRB, 100 Health Ave, Metropolis, CA 90210
- Subinvestigators: David Chen, MD; Sarah Jenkins, RN, PhD
- Signature Date: 05/15/2026
What this filled form sample shows:
- Accurate identification of the investigator and the clinical site (Section 1 and 2).
- Clear specification of the clinical protocol and investigational drug (Section 3).
- Correct listing of the Institutional Review Board (IRB) responsible for study oversight (Section 5).
- Properly documented subinvestigators who will assist in the conduct of the study (Section 6).
- Signed commitment to conduct the study in accordance with federal regulations (Section 9).
Form specifications and details:
| Form Name: | FDA Form 1572, Statement of Investigator |
| Use Case: | Investigational New Drug (IND) Clinical Trial |
| Purpose: | To provide the sponsor with information about the investigator's qualifications and the clinical site, and to obtain a commitment from the investigator to comply with FDA regulations for clinical investigations. |
Created: February 13, 2026 01:20 AM